THÔNG TIN CHUNG
Thông tin tổng quát
1.1 Tên đề tài: Xúc tác cho phản ứng reforming methane: Ảnh hưởng của hàm lượng chất xúc tiến và các thông số nhiệt động đến quá trình phản ứng
1.3 Danh sách chủ trì, thành viên tham gia thực hiện đề tài
(học hàm, học vị) Đơn vị công tác Vai trò thực hiện đề tài
01 Trần Ngọc Thắng, Thạc sĩ Khoa Công nghệ Hóa học, Đại học Công nghiệp TP HCM
02 Phạm Hoàng Ái Lệ, Thạc sĩ Khoa Công nghệ Hóa học, Đại học Công nghiệp TP HCM
1.5.1 Theo hợp đồng: từ tháng 03 năm 2021 đến tháng 03 năm 2022
1.5.2 Gia hạn (nếu có): không
1.5.3 Thực hiện thực tế: từ tháng 03 năm 2021 đến tháng 03 năm 2022
1.6 Những thay đổi so với thuyết minh ban đầu (nếu có): Không
1.7 Tổng kinh phí đƣợc phê duyệt của đề tài: 55 triệu đồng.
Kết quả nghiên cứu
Khí tổng hợp là nguyên liệu quan trọng trong sản xuất nhiên liệu tổng hợp qua phản ứng Fischer-Tropsch và methanol Có ba phương pháp chính để sản xuất khí tổng hợp: khí hóa than đá, oxy hóa một phần khí thiên nhiên và reforming khí thiên nhiên bằng hơi nước Tuy nhiên, những phương pháp này tạo ra lượng lớn khí CO2, góp phần vào hiệu ứng nhà kính Do đó, phát triển phương pháp sản xuất khí tổng hợp không phát sinh CO2 là cần thiết để bảo vệ môi trường Reforming methane bằng CO2 được coi là giải pháp bền vững cho ngành công nghiệp hóa chất Kim loại cobalt, với tính chất bền nhiệt và khả năng hoạt động cao trong phản ứng reforming methane, là một xúc tác tiềm năng cho các quá trình công nghiệp, mặc dù vẫn gặp phải vấn đề giảm hoạt tính do cặn carbon.
Nghiên cứu này khảo sát vai trò của chất xúc tiến lathanium trong xúc tác cobalt trên Al2O3 và ảnh hưởng của các thông số động học đến phản ứng reforming methane Tính chất xúc tác được đánh giá trước và sau phản ứng qua các phương pháp như XRD, TPR, phân tích hấp phụ/giải hấp N2 và Raman Hoạt tính xúc tác được kiểm tra trong hệ phản ứng tầng cố định, trong khi dữ liệu động học được phân tích bằng phần mềm Polymath để xác định cơ chế phản ứng.
2 Mục tiêu a) Mục tiêu tổng quát
Nghiên cứu này tập trung vào việc tổng hợp hệ xúc tác cobalt trên chất mang có cấu trúc xốp thông qua phương pháp bay hơi dung môi tự hình thành cấu trúc (EISA) Đồng thời, tác giả cũng đánh giá hoạt tính xúc tác của hệ này trong phản ứng reforming methane bằng CO2.
Hệ xúc tác được thiết kế bao gồm chất mang Al2O3 có cấu trúc xốp trung bình, chất xúc tác cobalt và chất xúc tiến lathanium oxit Nghiên cứu này tập trung vào việc khảo sát ảnh hưởng của hàm lượng chất xúc tiến đến hoạt tính xúc tác trong phản ứng reforming methane bằng CO2.
Khảo sát tác động của áp suất riêng phần của tác chất lên độ chuyển hóa, hiệu suất và sự hình thành carbon trên bề mặt xúc tác, từ đó xây dựng mô hình động học cho phản ứng.
Nội dung 1: Phân tích, đánh giá tình hình nghiên cứu trong và ngoài nước về phản ứng reforming methane bằng CO 2
- Cách tiếp cận: Xuất phát từ nhu cầu thực tế tại Việt Nam để đề xuất các phương pháp nghiên cứu giải quyết vấn đề
Báo cáo tổng hợp đánh giá tình hình nghiên cứu, nêu rõ mục tiêu và tính cấp thiết của đề tài, đồng thời tổng quan các công trình nghiên cứu liên quan trong và ngoài nước.
Nội dung 2: Tổng hợp xúc tác xLa-10%Co/Al 2 O 3 (x: 0 – 8 %)
Chúng tôi áp dụng các kỹ thuật tổng hợp hiện có để phát triển vật liệu có cấu trúc trung bình, phục vụ làm chất mang cho xúc tác Đồng thời, chúng tôi lựa chọn các nguyên liệu có tiềm năng ứng dụng trong quy mô công nghiệp nhằm hình thành hệ xúc tác cho phản ứng reforming methane bằng CO2.
- Kết quả: Mẫu xúc tác có cấu trúc xốp trung bình và hạt kim loại có kích thước nano
Nội dung 3: Đánh giá tính chất hóa lý của xúc tác xLa-10%Co/Al 2 O 3 (x: 0 – 8 %)
Để đánh giá hoạt tính cho phản ứng reforming methane bằng CO2, cần xem xét các tính chất quan trọng như kích thước phân tử cobalt, tính chất xốp của hệ xúc tác, khả năng khử của xúc tác, cũng như tính acid/bazo của xúc tác.
- Kết quả: Tính chất của các xúc tác, mối liên quan giữa hàm lƣợng chất xúc tiến và tính chất hóa lý của hệ xúc tác đƣợc làm rõ
Nội dung 4: Đánh giá hoạt tính của xúc tác xLa-10%Co/Al 2 O 3 (x: 0 – 8 %) cho phản ứng reforming methane bằng CO 2
- Cách tiếp cận: Hoạt tính của xúc tác đƣợc đánh giá dựa trên độ chuyển hóa của tác chất
(CH 4 , CO 2 ), hiệu suất hình thành sản phẩm (H2, CO) và tỉ lệ thành phần H2/CO
Kết quả nghiên cứu cho thấy hoạt tính của các xúc tác trong phản ứng reforming methane được đánh giá và so sánh, từ đó rút ra mối quan hệ giữa hàm lượng chất xúc tác và khả năng hoạt động của chúng.
Nội dung 5: Đánh giá tính chất của các xúc tác sau quá trình sử dụng cho phản ứng reforming methane bằng CO 2
Các tính chất ảnh hưởng đến thời gian hoạt động của xúc tác bao gồm hàm lượng carbon hình thành, loại carbon hình thành và hình thái của carbon hình thành Những yếu tố này được đánh giá chi tiết thông qua các phân tích hiện đại.
- Kết quả: Kết quả phân tích các xúc tác sau quá trình phản ứng reforming methane bằng
Đánh giá ảnh hưởng của áp suất riêng phần CO2 và CH4 đến phản ứng reforming methane là rất quan trọng, đặc biệt khi sử dụng xúc tác với hàm lượng chất xúc tiến tối ưu khác nhau Nghiên cứu này giúp hiểu rõ hơn về cách các yếu tố này tác động đến hiệu suất và hiệu quả của quá trình reforming methane, từ đó cải thiện công nghệ và ứng dụng trong ngành năng lượng.
Sự thay đổi của điều kiện nhập liệu ảnh hưởng đến khả năng làm việc của xúc tác trong phản ứng reforming methane bằng CO2 Nghiên cứu này nhằm phân tích phạm vi hoạt động của xúc tác để đánh giá hiệu quả và tiềm năng ứng dụng trong quá trình reforming.
- Kết quả: Kết quả đánh giá hoạt tính của xúc tác khi thay đổi các điều kiện nhập liệu
Nội dung 7: Nghiên cứu cơ chế của phản ứng reforming methane trên xúc tác với hàm lƣợng chất xúc tiến tối ƣu
Cơ chế phản ứng reforming methane trên xúc tác được làm sáng tỏ thông qua các mô hình động học như định luật Power Law và mô hình Langmuir–Hinshelwood, giúp xác định hàm lượng chất xúc tiến tối ưu cho quá trình này.
- Kết quả: Kết quả mô tả cơ chế phản ứng reforming methane bằng CO2 trên xúc tác với hàm lƣợng chất xúc tiến tối ƣu
Nội dung 8: Viết báo cáo và bài báo tổng kết
- Cách tiếp cận: Dựa trên kết quả nghiên cứu, viết báo cáo phân tích và công bố trên tạp chí chuyên ngành có uy tín
- Kết quả: Bài báo công bố trên tạp chí ISI
4 Tổng kết về kết quả nghiên cứu Đề tài đã đạt đƣợc các kết quả nghiên cứu sau:
Khí tổng hợp đóng vai trò quan trọng trong ngành công nghiệp hóa chất, với nhiều ứng dụng đa dạng Bài viết này sẽ phân tích và đánh giá hoạt tính, cũng như những ưu nhược điểm của các loại xúc tác được sử dụng trong phản ứng reforming methane để sản xuất khí tổng hợp.
Nghiên cứu đã chỉ ra rằng chất xúc tiến Lanthanum có ảnh hưởng đáng kể đến các tính chất hóa lý của xúc tác Cobalt, đồng thời khảo sát hoạt tính xúc tác của nó trong phản ứng reforming methane.
Sản phẩm đề tài, công bố và kết quả đào tạo
3.1 Kết quả nghiên cứu (sản phẩm dạng 1,2,3)
Yêu cầu khoa học hoặc/và chỉ tiêu kinh tế - kỹ thuật Đăng ký Đạt đƣợc
1 Bài báo khoa học 01 bài tạp chí ISI 01 bài tạp chí ISI
3.2 Kết quả đào tạo (Không có)
IV Tình hình sử dụng kinh phí
2 Nguyên, nhiên vật liệu, cây con… 50.000.000 50.000.000
6 Hội nghị, hội thảo, thù lao nghiệm thu giữa kỳ
7 In ấn, Văn phòng phẩm 2.437.200 2.437.200
V Kiến nghị Đề tài đƣợc thực hiện tại khoa Công nghệ hóa học trên hệ thống thiết bị phản ứng dòng vi lƣợng Các kết quả phân tích đƣợc thực hiện trên các thiết bị hiện có của Khoa
Công nghệ hóa học và sự hợp tác với các trung tâm nghiên cứu quốc tế đóng vai trò quan trọng trong thành công của các đề tài nghiên cứu Điều này nhấn mạnh tầm quan trọng của việc phối hợp trong nghiên cứu khoa học Để thúc đẩy hiệu quả hơn nữa, cần có cơ chế tài chính cụ thể từ nhà trường cho các dự án hợp tác giữa các cơ sở nghiên cứu trong và ngoài nước.
VI Phụ lục sản phẩm
Sản phẩm của đề tài là 01 (Một) bài báo khoa học đăng trên tạp chí ISI có chỉ số IF 2,79
- Tên tạp chí/Nhà xuất bản: Topic in Catalysis/Springer
- Tên bài báo: CO 2 Reforming of CH 4 on Mesoporous Alumina‑Supported Cobalt Catalyst: Optimization of Lanthana Promoter Loading (2021)
TP HCM, ngày tháng năm
Chủ nhiệm đề tài Phòng QLKH&HTQT (ĐƠN VỊ)
Trưởng (đơn vị) (Họ tên, chữ ký)
Kiến nghị
Đề tài nghiên cứu được thực hiện tại Khoa Công nghệ Hóa học, sử dụng hệ thống thiết bị phản ứng dòng vi lượng Các kết quả phân tích được tiến hành trên các thiết bị hiện có của Khoa, đảm bảo tính chính xác và hiệu quả trong quá trình nghiên cứu.
Công nghệ hóa học tại các trung tâm nghiên cứu trong nước đã đạt được thành công nhờ sự hợp tác với các nhà nghiên cứu nước ngoài, điều này cho thấy tầm quan trọng của sự phối hợp trong nghiên cứu khoa học Để thúc đẩy hơn nữa các đề tài và dự án chung giữa các cơ sở nghiên cứu trong và ngoài nước, nhà trường cần có cơ chế tài chính cụ thể hỗ trợ cho các hoạt động này.
Phụ lục sản phẩm
Sản phẩm của đề tài là 01 (Một) bài báo khoa học đăng trên tạp chí ISI có chỉ số IF 2,79
- Tên tạp chí/Nhà xuất bản: Topic in Catalysis/Springer
- Tên bài báo: CO 2 Reforming of CH 4 on Mesoporous Alumina‑Supported Cobalt Catalyst: Optimization of Lanthana Promoter Loading (2021)
TP HCM, ngày tháng năm
Chủ nhiệm đề tài Phòng QLKH&HTQT (ĐƠN VỊ)
Trưởng (đơn vị) (Họ tên, chữ ký)
BÁO CÁO CHI TIẾT ĐỀ TÀI NGHIÊN CỨU KHOA HỌC
TỔNG QUAN
1.1 Tổng quan vấn đề nghiên cứu
Khí tổng hợp, bao gồm hỗn hợp H2 và CO, là nguyên liệu quan trọng trong ngành công nghiệp hóa dầu, đóng vai trò chính trong sản xuất nhiên liệu tái tạo qua phản ứng Fischer-Tropsch và sản xuất methanol, nhằm thay thế nhiên liệu hóa thạch Các ứng dụng của khí tổng hợp được thể hiện trong Hình 1, trong khi quy mô thị trường của nó được trình bày trong Bảng 1.
Hình 1 Các ứng dụng của syngas theo tỉ lệ mol H 2 /CO
Bảng 1 Quy mô thị trường thế giới của Syngas
Sản phẩm cuối Thị trường, 2016
Khí hóa than đá và oxy hóa một phần có xúc tác, cùng với steam reforming khí tự nhiên, đang trở thành những phương pháp phổ biến để sản xuất khí tổng hợp.
Steam reforming là phương pháp chủ yếu để sản xuất khí tổng hợp từ hydrocacbon, với quá trình diễn ra ở nhiệt độ từ 700 đến 1100°C trên chất xúc tác niken.
Khoảng 50% sản lượng hydro toàn cầu được sản xuất thông qua quá trình reforming hơi nước từ methane, và nhu cầu về hydro đang gia tăng mạnh mẽ do vai trò quan trọng của nó trong nhiều lĩnh vực.
Quá trình reforming hơi nước methane gặp một số hạn chế, bao gồm: cần lượng hơi nước quá nhiệt để ngăn chặn sự hình thành than cốc, yêu cầu năng lượng cao do bản chất thu nhiệt của phản ứng, chi phí vận hành cao và tỷ lệ H2/CO không phù hợp cho tổng hợp Fischer-Tropsch Do đó, nhiều kỹ thuật cải tiến đã được nghiên cứu trong những năm gần đây.
Quá trình oxy hóa một phần là một phương pháp được thể hiện trong phương trình 2 n m 2 2
Hiệu suất của phản ứng này không đạt kỳ vọng do một phần nguyên liệu thô đã bị đốt cháy hoàn toàn, dẫn đến sự hình thành CO2 và H2O Quá trình oxy hóa một phần có thể được cải thiện để tối ưu hóa kết quả.
Phương pháp TPOX được thực hiện ở nhiệt độ trên 1200°C, tùy thuộc vào tỷ lệ oxy-nhiên liệu, trong khi phương pháp CPOX hoạt động ở nhiệt độ khoảng 800°C - 900°C Cả hai phương pháp đều sử dụng hơi nước bổ sung để ổn định nhiệt độ phản ứng và giảm thiểu sự hình thành than cốc CPOX thích hợp cho nhiên liệu có nồng độ lưu huỳnh dưới 50 ppm, trong khi TPOX được áp dụng cho các vật liệu có hàm lượng lưu huỳnh cao hơn Tuy nhiên, nhược điểm lớn của cả hai phương pháp là sự không ổn định nhiệt độ và hiện tượng hình thành than cốc cũng như điểm nóng.
Việc phát thải quá mức CO2 và các sản phẩm không mong muốn từ các quá trình công nghiệp đang gây ra mối lo ngại về môi trường và hiệu ứng nhà kính Do đó, quá trình reforming CO2 của methane (CRM) đã trở thành một phương pháp tiềm năng để sản xuất khí tổng hợp, sử dụng CO2 và CH4 làm nguyên liệu Quá trình này không chỉ giúp hài hòa tài nguyên mà còn kết hợp công nghệ tiên tiến và bảo vệ môi trường, hướng tới một kịch bản bền vững cho ngành công nghiệp hóa dầu trong tương lai.
CO 2 với một hydrocacbon để tạo ra khí tổng hợp được trình bày trong Phương trình 3
Methane dry reforming (CRM) đang thu hút sự chú ý đáng kể trong ngành công nghiệp năng lượng Thuật ngữ “dry” trong khái niệm reforming này ám chỉ việc thay thế nước bằng carbon dioxide trong quá trình chuyển đổi methane thành hydrogen và carbon Phương pháp này không chỉ giúp giảm lượng khí thải CO2 mà còn tối ưu hóa việc sử dụng nguồn năng lượng tái tạo.
So với phương pháp reforming bằng hơi nước, CRM mang lại nhiều lợi ích về năng lượng do yêu cầu ít năng lượng hơn cho quá trình bay hơi nước Hỗn hợp sản phẩm với tỷ lệ H2/CO thấp có thể được sử dụng trực tiếp trong tổng hợp Fischer-Tropsch Mặc dù phương pháp này có những lợi ích về môi trường và kinh tế, nhưng chi phí cao và độ ổn định hoạt động thấp của chất xúc tác đã hạn chế khả năng ứng dụng quy mô lớn Do đó, việc thiết kế chất xúc tác hiệu quả về chi phí, có hoạt tính cao và giảm thiểu sự hình thành than cốc là ưu tiên hàng đầu trong ngành CRM, dẫn đến nhiều nghiên cứu và công bố trong những năm gần đây.
1.2 Sự phát triển chất xúc tác
Trong sứ mệnh thương mại hóa sản xuất khí tổng hợp qua CRM, việc phát triển các chất xúc tác giá rẻ, bền vững và hiệu suất cao là rất quan trọng Động lực chính của quá trình CRM là sự tồn tại các vị trí hoạt động để phân ly CH4 và CO2.
Tính bazo của chất xúc tác đóng vai trò quan trọng trong việc tăng tốc độ hấp thụ hóa học và hoạt hóa, đồng thời giúp phân hủy khí axit CO2 khi có mặt của pha kim loại hoạt động
Các vị trí trống oxy trên chất mang được hình thành khi các nguyên tử oxy được giải phóng trong môi trường khử và sau đó được lưu trữ trong điều kiện oxy hóa Những khuyết tật oxy này có vai trò quan trọng trong việc cắt đứt liên kết C-O để kích hoạt CO2, từ đó tạo ra một nhóm nguyên tử oxy trên bề mặt chất xúc tác Các loại oxy này giúp ngăn chặn sự khử hoạt tính của chất xúc tác bằng cách oxy hóa carbon ban đầu, vốn đã được định hướng để lắng đọng.
Các tương tác mạnh mẽ giữa chất mang và chất xúc tác kim loại là yếu tố quan trọng để nâng cao hoạt tính xúc tác và khả năng chống lại quá trình khử hoạt tính ở nhiệt độ phản ứng cao Tính chất của chất xúc tác phụ thuộc vào mức độ và cách thức tương tác giữa các thành phần cấu thành Để duy trì sự ổn định của kim loại và ngăn ngừa sự kết tụ với các tinh thể kim loại lân cận, cần thiết phải áp dụng nhiệt độ cao nhằm hạn chế quá trình thiêu kết kim loại.
Các kim loại quý như rhodi (Rh), ruthenium (Ru), iridium (Ir), palladium (Pd) và platinum (Pt) là những chất xúc tác điển hình cho phản ứng CRM, với hoạt tính xúc tác cao và khả năng chống lại sự hình thành cacbon tốt hơn các kim loại chuyển tiếp khác Trong số đó, Rh và Ru trên chất mang thể hiện hoạt tính vượt trội so với Ir, Pd
NGUYÊN LIỆU VÀ CÁC PHƯƠNG PHÁP NGHIÊN CỨU
Aluminum oxide (Al2O3) was synthesized by dissolving 0.98 g of tri-block copolymer Pluronic® P-123 (EO 20 PO 70 EO 20, average molecular weight 5800, Sigma-Aldrich, St Louis, Missouri, US) in a solvent mixture of 14.7 ml containing 25% water and 75% ethanol, using a magnetic stirrer The solution was vigorously mixed for 30 minutes at 303 K, after which 3.68 g of aluminum nitrate nonahydrate (Al(NO3)3·9H2O, 98%, Merck Millipore) and 1.6 ml of hydrochloric acid (37%, Merck Millipore) were added and stirred continuously for an additional hour Hydrothermal treatment was conducted in an autoclave for 24 hours at 373 K, followed by gradual evaporation of the obtained mixture.
The gel-like substance was heated in a Memmert UF1060 oven at 333 K for 48 hours in Schwabach, Germany Subsequently, it was transferred to a Carbolite CWF 1200 furnace in Sheffield, England, where it underwent a sintering process at 1073 K for 5 hours, with a heating rate of 1 K/min, to produce the Al2O3 support material.
Trong các nghiên cứu trước đây, thành phần tối ưu cho chất xúc tiến và kim loại hoạt động được xác định là 3% và 10% Vì vậy, hàm lượng này đã được áp dụng lại trong nghiên cứu hiện tại.
Hợp chất 3%La-10%Co/Al2O3 được tổng hợp thông qua phương pháp đồng tẩm ướt, sử dụng dung dịch 0,57 g Co(NO3)2.6H2O và 0,11 g La(NO3)3.6H2O trong 0,5 ml etanol khan, trộn với 1 g Al2O3 trong 1 giờ Sau khi ngâm tẩm, chất rắn được làm khô ở nhiệt độ 373 K qua đêm và nung ở 873 K trong 5 giờ với tốc độ gia nhiệt 1 K/min Phương pháp tẩm này cũng áp dụng để tổng hợp 10%Co/Al2O3 mà không cần bổ sung dung dịch xúc tiến.
Nhiễu xạ tia X được phân tích bằng máy quang phổ Miniflex 600 (Rigaku, Tokyo, Nhật Bản) sử dụng nguồn bức xạ Cu (λ = 1,5418 Å) Các mẫu nhiễu xạ được ghi lại trong phạm vi quét từ 3-80 độ với bước quét 0,02 độ và tốc độ quét 1 độ/phút Để xác định các đỉnh, cơ sở dữ liệu tiêu chuẩn (JCPDS) được tham khảo, và kích thước tinh thể được tính toán theo phương trình Scherrer dựa trên đỉnh có cường độ cao nhất.
trong đó θ là góc Bragg và β là độ rộng ở một nửa cường độ cực đại
Thiết bị hấp phụ khí tự động Tristar II 3020 của Micrometrics, USA, sử dụng nitơ hóa lỏng ở nhiệt độ 77 K để kiểm tra đặc tính kết cấu của mẫu Trước khi tiến hành thử nghiệm, mẫu thử được khử khí trong dòng N2 ở nhiệt độ 573 K trong 1 giờ nhằm loại bỏ tạp chất và hơi ẩm.
Quá trình khử theo chương trình nhiệt H2 (H2-TPR) được thực hiện trên thiết bị AutoChem II-2920 (Micromeritics, Georgia, Mỹ) Mỗi lần chạy sử dụng khoảng 50 mg chất xúc tác, được đặt ở trung tâm của ống chữ U bằng thạch anh Quá trình bắt đầu bằng cách thổi N2 ở nhiệt độ 373 K trong 30 phút, sau đó chuyển sang bước khử với dòng khí 10% H2/N2 (50 ml/phút) và nhiệt độ được lập trình từ 373 K.
K đến 1173 K tốc độ 10 K min -1 Mẫu khử đƣợc giữ không đổi ở 1173 K trong 30 phút trước khi hạ nhiệt xuống nhiệt độ môi trường trong N 2
Quá trình oxy hóa TPO đƣợc thực hiện trên thiết bị TGA Q500 của (TA Instruments, Newcastle, DE, US) Đầu tiên, mẫu thử được khử nước ở 373 K trong dòng N2 (100 ml min -
1) trong 30 phút Sau đó, mẫu đƣợc tiếp xúc với dòng hỗn hợp của 20 ml O 2 min -1 và 80 ml min -1 N 2 trong khi nhiệt độ đƣợc tăng từ 373 lên 1023 K với tốc độ 10 K min -1 , tiếp theo là quá trình oxy hóa đẳng nhiệt ở 1023 K trong 30 phút
Quang phổ Raman được thực hiện bằng máy quang phổ NRS-3100 (JASCO, Tokyo, Nhật Bản) ở nhiệt độ phòng, sử dụng nguồn kích thích laser sơ cấp ở bước sóng 532 nm Kính hiển vi điện tử có độ phân giải cao (HRTEM) được thực hiện bằng kính hiển vi TOPCOM EM-002B (Nhật Bản) với điện áp 200 kV.
2.3 Đánh giá hoạt tính xúc tác
Các chất xúc tác được khảo sát cho quá trình CRM ở nhiệt độ từ 973 đến 1073 K và áp suất 0,1 MPa Mỗi thí nghiệm sử dụng khoảng 0,1 g chất xúc tác được đặt vào trung tâm lò phản ứng tầng cố định có chiều dài 17 inch và đường kính 3/8 inch, được cố định bằng len thạch anh Tốc độ phân phối của chất phản ứng và khí pha loãng nitơ được điều chỉnh riêng biệt bằng bộ điều khiển lưu lượng khối lượng (Alicat Scientific, Tucson, AZ, USA) Tất cả các khí đã được trộn đều trước khi vào lò phản ứng, với tổng vận tốc trong không gian được giữ cố định ở mức 36 L g cat -1 h -1 cho mọi lần chạy.
Trước khi tiến hành đánh giá xúc tác, quá trình hoạt hóa H2 được thực hiện ở nhiệt độ 1073 K trong 1 giờ với hỗn hợp khí 50% H2/N2 (60 ml/phút) Tất cả sản phẩm khí thoát ra từ lò phản ứng được ghi lại bằng đầu dò dẫn nhiệt (TCD) trong Sắc ký khí Agilent 6890 (Agilent Technologies, Santa Clara, CA, USA) Các chỉ số phản ứng chính, đặc biệt là độ chuyển hóa, được theo dõi và phân tích kỹ lưỡng.
Hiệu suất của CO và H2, cùng với tỷ lệ H2/CO, được ước tính dựa trên công thức tương ứng, sử dụng tốc độ dòng vào (Q in) và dòng ra (Q out) tính bằng mol/s.
KẾT QUẢ VÀ THẢO LUẬN
3.1 Đánh giá thuộc tính chất xúc tác
Các tính chất vật lý của Al2O3 tinh khiết và chất xúc tác được trình bày trong Bảng 4, cho thấy diện tích bề mặt riêng BET và tổng thể tích lỗ rỗng của Al2O3 lần lượt là 173,4 m²/g và 0,28 cm³/g Các giá trị này tương đương với Al2O3 thương mại như Sasol Puralox SCCa-150/200 (175,3 m²/g, 0,46 cm³/g) và Al2O3 (Brockmann I) từ Sigma-Aldrich Chemicals (174,1 m²/g, 0,38 cm³/g) Đối với 10%Co/Al2O3, diện tích bề mặt khoảng 141,9 m²/g, và giá trị BET giảm xuống 107,9 m²/g khi hàm lượng La tăng lên trong mẫu 8%La-10%Co/Al2O3 Việc bổ sung chất xúc tác cho thấy sự kết hợp thành công của La2O3 với chất mang Al2O3, dẫn đến sự giảm diện tích bề mặt.
Bảng 4 Các thuộc tính vật lý của chất mang và xúc tác
Thể tích lỗ xốp tổng (cm 3 g -1 ) Đường kính lỗ trung bình (nm)
Kích thước tinh thể Co3O4 được xác định qua phương trình Scherrer với góc 2θ là 37,03° Đường cong giải hấp phụ N2 của các xúc tác Al2O3, 10%Co/Al2O3 và x%La2O3 10%Co/Al2O3 thể hiện trong Hình 11 Tất cả các đường hấp phụ/giải hấp đều thuộc loại IV theo phân loại IUPAC, với các vòng trễ H1 rõ ràng xuất hiện ở áp suất tương đối P/P0 khoảng 0,5 đến 0,9 Những đặc tính này cho thấy vật liệu có cấu trúc xốp mesoporous hoặc đặc điểm lỗ rỗng hình trụ đồng nhất (Tran et al., 2020).
Hình 11 Đường đẳng nhiệt hấp phụ/giải hấp N 2 của Al 2 O 3 , 10%Co/Al 2 O 3 và 10%Co/Al 2 O 3 xúc tiến bởi La với các hàm lƣợng khác nhau
So với chất mang Al2O3, các đường hấp phụ/giải hấp đẳng nhiệt của cả xúc tác được xúc tiến và không được xúc tiến có hình dạng tương tự Sự thay đổi không đáng kể về đường kính lỗ xốp trung bình từ 5,9 đến 6,5 nm cho thấy cấu trúc của chất mang Al2O3 không bị thay đổi trong quá trình mang kim loại lên Các hạt nano La2O3 và Co3O4 được phân tán mịn.
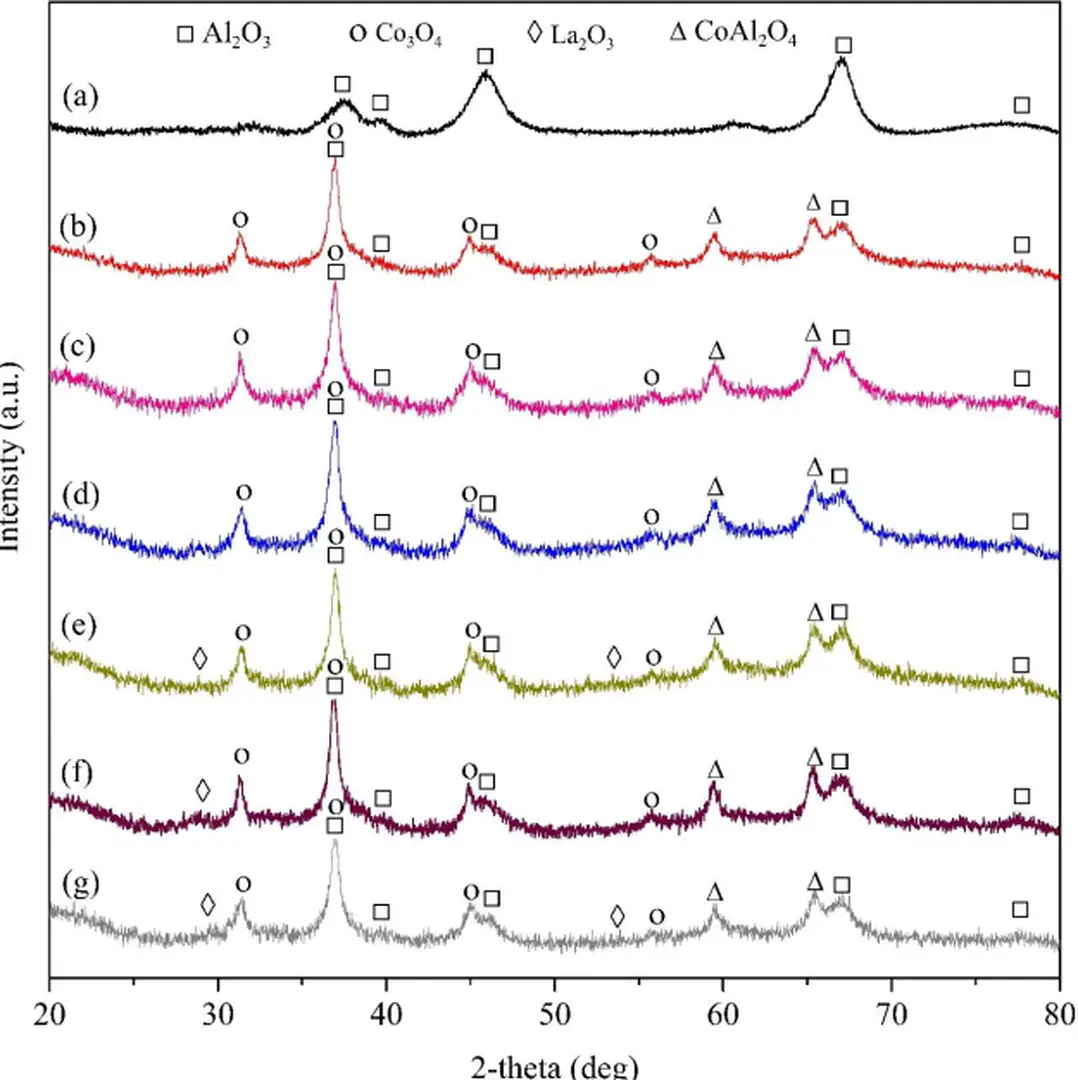
Phổ nhiễu xạ tia X (XRD) của chất mang và chất xúc tác được trình bày trong Hình 12 Chất mang Al2O3 (Hình 12 (a)) có các đỉnh 2θ đặc trưng tại 37,4°, 39,6°, 46,0°, 67,0° và 77,1° theo JCPDS số 04-0858, cho thấy sự xác định rõ ràng của nó (Feng, Zhang, Fang, Li, & Wang, 2016).
Trong nghiên cứu của Tran et al (2020), các mẫu xúc tác cho thấy sự hiện diện rõ ràng của các pha Co3O4 và CoAl2O4 Cụ thể, tín hiệu của pha Co3O4 được phát hiện tại các góc 31,3°, 37,0°, 44,9° và 55,8° (theo JCPDS số 74-2120), trong khi cobalt aluminate CoAl2O4 đặc trưng bởi các đỉnh tại 59,6° và 65,4° (theo JCPDS số 82-2246), cho thấy sự liên kết mạnh mẽ giữa chất mang γ-Al2O3 và kim loại CoO (Fayaz et al., 2019) Ngoài ra, các tinh thể La2O3 có đặc trưng tại 2θ: 29,9° và 53,4° (theo JCPDS số).
83-1355) (Osorio-Vargas, Campos, Navarro, Fierro, & Reyes, 2015; Shafiqah et al., 2020) không đƣợc phát hiện trên các mẫu có tải lƣợng chất xúc tiến thấp là 2% và 3% (xem Hình
Hàm lượng La cao hơn 4-8% dẫn đến sự xuất hiện các peak với cường độ thấp (Hình 12 (e) - (g)) Quan sát cho thấy, hạt nano La2O3 phân bố trên bề mặt chất xúc tác với kích thước cực nhỏ Ở hàm lượng thấp khoảng 2-3%, kích thước tinh thể của La2O3 sẽ nằm ngoài giới hạn phát hiện XRD (Campos, Osorio-Vargas, Flores-González, Fierro, & Reyes, 2016; Kondrat et al., 2018).
Hình 12 Cấu hình XRD của (a) Al 2 O 3 , (b) 10%Co/Al 2 O 3 , (c) 2%La-10%Co/Al 2 O 3 , (d) 3%La-10%Co/Al 2 O 3 , (e) 4%La-10%Co/Al 2 O 3 , (f) 5%La-10%Co/Al 2 O 3 , và (g) 8%La-10%
Kích thước tinh thể Co3O4 trong các chất xúc tác được tóm tắt trong Bảng 4, cho thấy khi không có chất xúc tiến, kích thước tinh thể là 10 nm Đặc biệt, khi bổ sung La2O3, kích thước tinh thể Co3O4 giảm đáng kể xuống còn 5,2-8,4 nm tùy thuộc vào hàm lượng Sự giảm kích thước này có thể do hiệu ứng pha loãng của La2O3, giúp cô lập các hạt Co3O4 và ngăn chặn sự kết tụ kim loại trong điều kiện nhiệt độ nung cao (Bahari et al., 2020).
3.1.3 Phân tích khử theo chương trình nhiệt độ H 2
Hình 13 minh họa tính chất khử H2-TPR của các chất xúc tác có và không có sự xúc tiến của La Trong tất cả các mẫu phân tích, ba đỉnh khử riêng biệt đã được quan sát Đặc biệt, hai đỉnh khử đầu tiên, được gọi là α và β, thể hiện quá trình khử hai bước của Co3O4 thành kim loại Co0 thông qua sự hình thành pha trung gian CoO (đỉnh α), sau đó được khử xuống pha Co0 (đỉnh β).
Tín hiệu rộng và không đáng kể (đỉnh γ) xuất hiện ở trên 1000 K được cho là do phản ứng khử cobalt aluminate thành kim loại Co 0 (Jean-Marie et al., 2011) Cường độ nhỏ của đỉnh γ cho thấy lượng CoAl2O4 tương đối nhỏ so với pha Co3O4 trong chất xúc tác.
Hình 13 Kết quả H 2 -TPR cho (a) 10%Co/Al 2 O 3 , (b) 3%La-10%Co/Al 2 O 3 , (c) 4%La-10%
Co/Al 2 O 3 , (d) 5%La -10%Co/Al 2 O 3 , và (e) 8%La-10%Co/Al 2 O 3
Sự suy giảm nhiệt độ khử của đỉnh α từ 768 K (10%Co/Al2O3) xuống 618 K (8%La-10%Co/Al2O3) khi hàm lượng La tăng từ 0% đến 8% cho thấy rõ ràng tác động của La2O3 Sự xúc tiến của La2O3 làm giảm quá trình khử Co3O4 thành CoO nhờ vào mật độ điện tử tăng cường trên bề mặt chất xúc tác, mà La2O3 hoạt động như một chất cho điện tử (Zhi, Guo, Wang, Jin, & Guo, 2011).
3.1.4 Giải hấp CO 2 theo chương trình nhiệt độ Để kiểm tra chức năng của chất xúc tiến La 2 O 3 trên tính bazo của xúc tác, phân tích
Nghiên cứu CO2-TPD đã được thực hiện trên các mẫu bao gồm chất mang Al2O3, 10%Co/Al2O3, 3%La-10%Co/Al2O3, 5%La-10%Co/Al2O3 và 8%La-10%Co/Al2O3 Kết quả cho thấy sự xuất hiện của một đỉnh rộng trong khoảng nhiệt độ từ 450 đến 950 K cho mỗi mẫu, điều này chỉ ra sự hiện diện của các tâm bazo mạnh trên bề mặt vật liệu (Shafiqah et al., 2020).
Lượng CO2 hấp phụ trên chất mang Al2O3 đạt khoảng 3,89 x 10^-2 mmol CO2 g cat^-1, trong khi giá trị này giảm xuống còn 3,62 x 10^-2 mmol CO2 g cat^-1 khi sử dụng 10% Co/Al2O3 Kết quả này phù hợp với nghiên cứu của Papageridis et al.
Việc kết hợp chất xúc tiến La2O3 với chất xúc tác 10%Co/Al2O3 đã dẫn đến việc hấp phụ một lượng CO2 lớn hơn, lên tới 7,72 x 10-2 mmol CO2 g cat-1, khi hàm lượng La2O3 được bổ sung từ 0 - 8% Sự bổ sung này đã nâng cao tính bazo của chất xúc tác, một yếu tố quan trọng trong CRM, giúp đảm bảo độ ổn định và hoạt tính của xúc tác.
Hình 14 Kết quả CO 2 -TPD của Al 2 O 3 , 10%Co/Al 2 O 3 , 3%La-10%Co/Al 2 O 3 , 5%La-
10%Co/Al 2 O 3 , và 8%La-10%Co/Al 2 O 3
3.2 Hoạt tính xúc tác cho CRM
Nghiên cứu CRM được thực hiện trên các chất xúc tác đã chuẩn bị để đánh giá ảnh hưởng của hàm lượng chất xúc tiến La đối với hoạt tính của chất xúc tác Kết quả cho thấy không có ảnh hưởng đáng kể từ trở lực truyền nhiệt và truyền khối trong phản ứng, với GHSV cố định là 36 L g cat -1 h -1 Phân tích cân bằng vật chất cho nguyên tố carbon cho thấy sai số không đáng kể, xác nhận độ chính xác của phép phân tích và thử nghiệm Độ chuyển hóa theo thời gian (TOS) của CH4 và CO2 ở nhiệt độ 1023 K thể hiện sự ổn định, cho thấy các chất xúc tác được xúc tiến và không được xúc tiến đều duy trì độ chuyển hóa CH4 ổn định.
La 2 O 3 tăng cường đáng kể chuyển hóa CH 4 từ 70,0% (10%Co/ Al 2 O 3 ) đến 90,5% (5%La- 10%Co/Al 2 O 3 ) như thể hiện trong Hình 15 Hành vi tương tự cũng được quan sát thấy đối với độ chuyển hóa CO 2 khi đƣợc xúc tiến La và chuyển hóa CO 2 đã tăng từ 77,1% lên 90,4% (xem Hình 16) Sự cải thiện của chuyển hóa CH 4 và CO 2 trên xúc tác La đƣợc cho là do kích thước tinh thể Co 3 O 4 nhỏ (Ayodele, Khan, Lam, & Cheng, 2016) và đặc tính bazo của chất xúc tiến La 2 O 3 tạo điều kiện hấp thụ CO 2 và khí hóa các loại carbon bề mặt, C x H y từ sự phân ly CH 4 (Sato, Takahashi, Kobune, & Gotoh, 2009) Do đó, chất xúc tác đƣợc xúc tiến La sẽ có ít cặn carbon hơn và cải thiện độ chuyển hóa so với chất xúc tác không đƣợc xúc tiến
KẾT LUẬN VÀ KIẾN NGHỊ
4.1 Kết luận Ảnh hưởng của chất xúc tiến La 2 O 3 và hàm lượng của nó đối với các tính năng hóa lý của chất xúc tác 10%Co/Al 2 O 3 cũng nhƣ hiệu suất của nó đối với phản ứng CRM đã đƣợc nghiên cứu Việc bổ sung các hạt nano La 2 O 3 về cơ bản không làm sai lệch cấu trúc mesoporous của chất mang Al 2 O 3
Cả hai oxit kim loại Co và La đều phân bố tốt trên bề mặt Al 2 O 3 với kích thước tinh thể Co 3 O 4 nhỏ trong khoảng 5,2-8,4 nm
Quá trình khử đƣợc giảm bớt (đối với Co 3 O 4 → CoO) và tăng nồng độ bazo của chất xúc tác đƣợc thể hiện rõ ràng khi kết hợp La 2 O 3
Nghiên cứu cho thấy rằng việc sử dụng tỷ lệ CH4/CO2 là 1:1 và nhiệt độ 1023 K đã dẫn đến sự gia tăng nồng độ tâm bazo, đồng thời giảm kích thước tinh thể của kim loại hoạt động Điều này liên quan đến việc xúc tiến bằng La2O3, giúp cải thiện hiệu suất chuyển hóa của CH4 và CO2 lần lượt đạt 29,3% và 17,3%.
Hình 24 TOS độ chuyển đổi chất phản ứng đạt đƣợc từ thử nghiệm độ bền 5%La-
10%Co/Al 2 O 3 ở 1023 K và tỷ lệ nhập liệu là 1
CHƯƠNG 4 KẾT LUẬN VÀ KIẾN NGHỊ
4.1 Kết luận Ảnh hưởng của chất xúc tiến La 2 O 3 và hàm lượng của nó đối với các tính năng hóa lý của chất xúc tác 10%Co/Al 2 O 3 cũng nhƣ hiệu suất của nó đối với phản ứng CRM đã đƣợc nghiên cứu Việc bổ sung các hạt nano La 2 O 3 về cơ bản không làm sai lệch cấu trúc mesoporous của chất mang Al 2 O 3
Cả hai oxit kim loại Co và La đều phân bố tốt trên bề mặt Al 2 O 3 với kích thước tinh thể Co 3 O 4 nhỏ trong khoảng 5,2-8,4 nm
Quá trình khử đƣợc giảm bớt (đối với Co 3 O 4 → CoO) và tăng nồng độ bazo của chất xúc tác đƣợc thể hiện rõ ràng khi kết hợp La 2 O 3
Nghiên cứu cho thấy việc sử dụng tỷ lệ CH4/CO2 là 1:1 và nhiệt độ 1023 K đã làm tăng nồng độ tâm bazo và giảm kích thước tinh thể của kim loại hoạt động Sự hiện diện của La2O3 đã cải thiện hiệu suất chuyển hóa CH4 và CO2, đạt được mức tăng tương ứng là 29,3% và 17,3%.
Sự xúc tiến của La2O3 có tác dụng ức chế đáng kể sự lắng đọng carbon, giảm từ 47,7% xuống còn 34,6% Điều này nhờ vào tính năng bazo của chất xúc tác và sự hình thành pha trung gian La2O2CO3, giúp loại bỏ các loại carbon bề mặt khỏi chất xúc tác trong quá trình CRM.
Trong số các chất xúc tác được nghiên cứu, 5%La-10%Co/Al2O3 nổi bật với khả năng kháng carbon, tạo ra CO và H2 hiệu quả nhất CRM thực hiện trên chất xúc tác này cho thấy sự hấp phụ liên kết kép tại chỗ của CH4 và CO2 qua phản ứng bề mặt hai phân tử Chất xúc tác cũng thể hiện độ ổn định tốt trong 48 giờ, với tỷ lệ chuyển đổi CO2 và CH4 giảm không đáng kể, lần lượt là 0,05% h-1 và 0,03% h-1.
Tỷ lệ H 2 /CO thu đƣợc là 0,84-0,98 thích hợp cho phản ứng Fischer-Tropsch để tạo ra nhiên liệu hydrocarbon lỏng
4.2 Kiến nghị Đề tài đƣợc thực hiện tại khoa Công nghệ hóa học trên hệ thống thiết bị phản ứng dòng vi lƣợng Các kết quả phân tích đƣợc thực hiện trên các thiết bị hiện có của Khoa Công nghệ hóa học và tại các trung tâm nghiên cứu trong nước Sự hợp tác của các cá nhân nghiên cứu nước ngoài cũng góp phần cho sự thành công của đề tài Điều đó chứng minh tầm quan trọng của việc phối hợp, hợp tác trong nghiên cứu khoa học Qua đó kiến nghị nhà trường có cơ chế cụ thể về mặt tài chính cho việc thực hiện các đề tài, dự án chung giữa 2 cơ sở nghiên cứu trong hoặc ngoài nước